What Does HACCP Stand For?
HACCP stands for Hazard Analysis and Critical Control Points. It is a globally recognized method for spotting, assessing, and controlling anything that could compromise food safety. In simple terms, it helps you prevent problems before they have a chance to disrupt your operation.
It covers the entire lifecycle of your product:
• Raw materials
• Processing
• Packaging
• Storage
• Distribution
• Final consumption
and It’s used by leading manufacturers, demanded by major buyers, and required across multiple regulatory systems worldwide.
What Is a HACCP Plan?
In short, a HACCP plan identifies food safety hazards, defines controls, and outlines how your facility prevents risks at each process step.
To unpack this further… HACCP plan is the structured, risk-based blueprint your operation uses to understand where food safety hazards can arise and how you prevent them. It evaluates every step of your production process, pinpoints biological, chemical, physical, radiological and allergen risks, and documents the exact limits, checks, and corrective actions needed to keep those risks under control.
Every HACCP plan is specific to your facility because no two food operations share the same ingredients, equipment, layout, or processing steps. It becomes the base of your entire food safety system by connecting how hazards are identified, how controls work, and how monitoring and verification prove that your process stays on track.
Importantly, a HACCP plan is not just “good practice.” It is a core requirement for GFSI-recognized certification programs such as BRCGS, SQF, and FSSC 22000, and is expected by major retailers and regulatory authorities worldwide. Without a functioning HACCP plan and system, you cannot meet global food safety standards or maintain customer confidence. The plan itself is the documented process, while a HACCP system is how your team executes it every day, performing CCP checks, recording data, managing deviations, calibrating equipment, and continuously verifying performance.
A strong HACCP plan brings clarity, structure, and control to your manufacturing environment, helping you prevent failures, reduce risk, and consistently deliver safe food at scale.
Why HACCP Matters in Food Manufacturing?
HACCP strengthens operational control, reduces risk, supports regulatory compliance, and improves process consistency across teams.
When HACCP becomes part of the daily rhythm, it shields your product and your brand, cuts down the chance of costly recalls, and keeps customers feeling confident. It helps every batch hit the same high safety mark no matter who is running the line, and it sets you up to meet global expectations, including programs like BRCGS, SQF and FSSC 22000. Above all, it brings order to busy production floors. It highlights what truly matters, shows exactly where teams should focus, and gives staff the clear steps they need to keep food safe even on the most hectic days.
HACCP Plan Step by Step, What Comes Before the 7 Principles?
Before you jump into the seven HACCP principles, your operation needs a solid foundation. This is the step many teams skip because it feels “obvious,” yet it’s one of the main reasons HACCP plans fall apart during audits. When the groundwork is weak, your hazard analysis becomes guesswork, CCPs get misclassified, and monitoring turns into chaos instead of control.
A strong HACCP plan starts with confirming how your facility actually works today. It must reflect how the process runs today, not how it was originally designed. This stage is about creating a shared, verified understanding of your products, your process, and the real-world conditions on the production floor.
Here’s what you need in place before the principles can do their job:
1. Build a Competent HACCP Team
You need the people who understand every corner of the operation. That usually means QA, production, sanitation, engineering, maintenance, warehousing, and supply chain. Each group sees different risks and will spot different weak points. A strong team prevents blind spots and keeps the plan practical instead of theoretical.
2. Align on Product and Process Requirements
Your team already knows the product, but HACCP requires that information to be aligned, documented, and usable for risk assessment. That includes intended use, storage conditions, processing characteristics, and factors that influence microbial, chemical, physical, or allergen risks. This alignment ensures everyone is working from the same playbook.
3. Create a Verified Process Flow
A HACCP plan only works if your process flow reflects reality, not wishful thinking. Map each step from receiving through distribution, then confirm that it matches reality. This is not busywork, it avoids the classic “paper process” that looks perfect until an auditor asks why your line operators are doing something completely different.
4. Verify the Process on the Production Floor
This is where the real hazards reveal themselves. A floor walkthrough shows the unpolished version of your operation: manual adjustments, temporary fixes, rework loops, bottlenecks, shortcuts, and subtle cross-contact risks. These details rarely show up in SOPs or flow maps, but they absolutely matter for your hazard analysis.
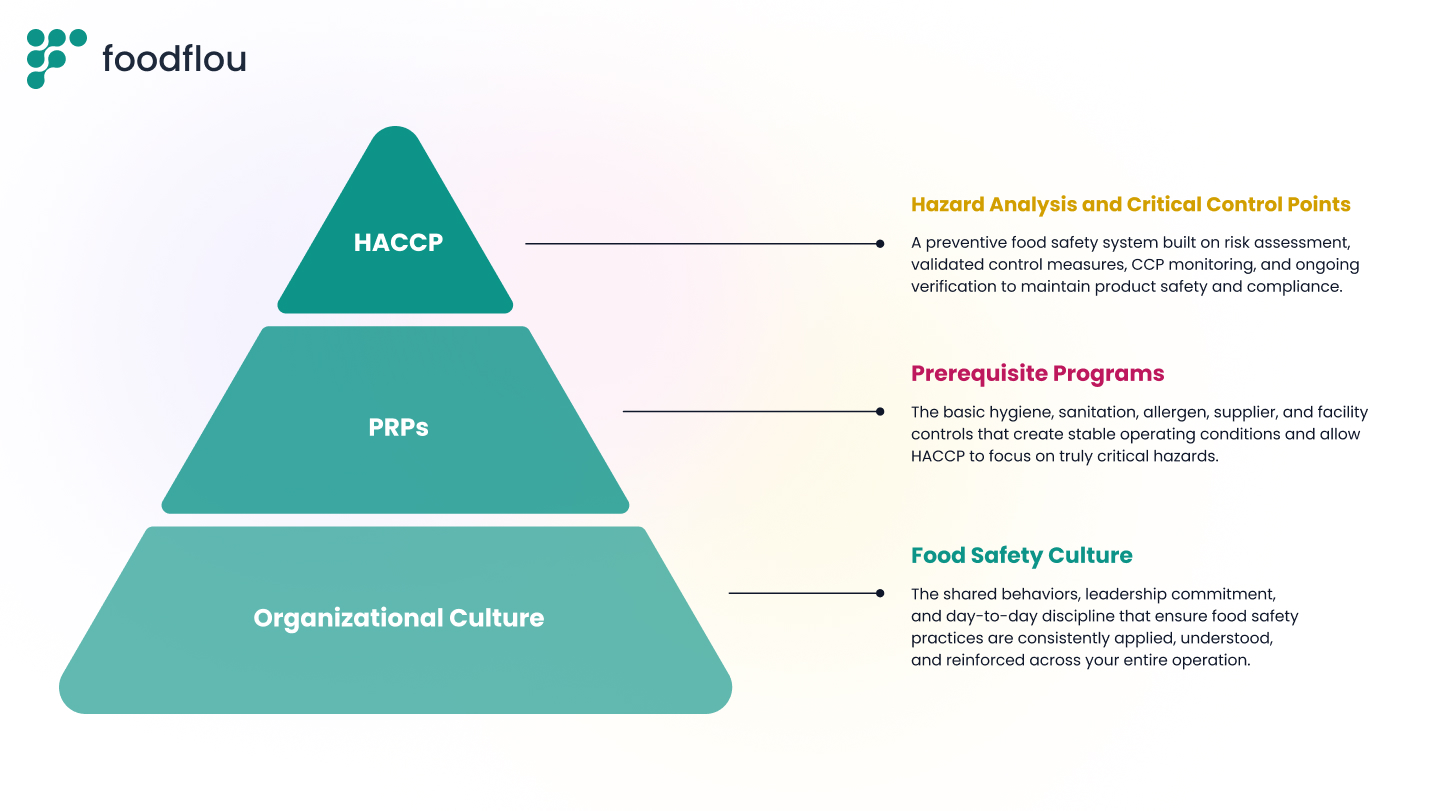
Food Safety Culture Comes First
Food safety begins with the behaviors and decisions inside your operation long before you get to hazard analysis or CCPs. Management commitment shapes everything, from how clean the facility stays to how confidently operators report deviations. A strong culture ensures people understand their role, follow procedures consistently, and make food safety part of daily work rather than something performed only during audits. HACCP only works when the culture supports it.
Review Your Prerequisite Programs
Prerequisite programs create the baseline conditions that keep everyday hazards controlled so your HACCP plan can focus on the critical risks.
Before a HACCP plan can do its job, the fundamentals of your facility must already be under control. These foundational programs handle the broad, recurring hazards that exist in every food operation, things like poor cleaning, pest activity, cross-contamination, untrained staff, or poorly maintained equipment. HACCP is designed to manage specific, high-impact hazards, not compensate for a facility that can’t maintain basic hygiene standards.
Think of prerequisite programs as the stability system that keeps your operation predictable. When these programs are strong, your HACCP plan becomes lean, focused, and effective. When they’re weak, HACCP gets overloaded, CCPs multiply unnecessarily, and audits turn into therapy sessions.
Below are the core prerequisite programs every food manufacturer should maintain, explained in practical terms.
1. Good Manufacturing Practices (GMPs)
GMPs define the conditions in which food must be produced. When done well, GMPs prevent 90% of “avoidable” food safety issues before HACCP even enters the conversation.
GMPs typically cover:
• Facility design: hygienic layout, controlled product flow, cleanable surfaces, protected water and air systems
• Personnel hygiene: proper attire, handwashing, restricted jewelry, illness reporting
• Operational discipline: controlled traffic, no eating or drinking in production, controlled movement between allergen and non-allergen zones
• Equipment maintenance: properly maintained machinery, no loose parts, no rough welds that trap residues
When GMPs are consistently applied, they reduce cross-contamination, improve line efficiency, and drastically cut down on low-level hazards.
2. Sanitation Standard Operating Procedures (SSOPs)
SSOPs document how your facility is cleaned, how often, by whom, and with what. They prevent microbial buildup, allergen carryover, and cross-contact that could otherwise escalate into CCP-level hazards.
Effective SSOPs include:
• detailed cleaning instructions for each piece of equipment
• chemical concentrations and contact times
• verification steps (ATP, swabs, visual checks)
• sanitation crew responsibilities
• and inspection sign-offs
If sanitation is inconsistent, HACCP becomes impossible to maintain, because you cannot control hazards in a dirty environment.
3. Supplier Control Programs
Unsafe raw materials will break even the best HACCP plan. Full visibility into your suppliers, including ingredients, packaging, processing aids, logistics partners, and external service providers, is essential for understanding the real risk entering your facility. A supplier control program ensures that everything coming into your operation is safe, verified, and aligned with your standards before it ever reaches production.
Supplier control programs cover:
• approved supplier lists
• specifications and COAs
• supplier audits and risk assessments
• ongoing performance monitoring
• defined acceptance and rejection criteria
A strong supplier program means you are not trying to correct problems inside your facility that should have been prevented long before the product arrived.
4. Pest Control
A pest control program prevents contamination from insects, rodents, and other unwelcome visitors. While not glamorous, it’s essential, and it's a top audit failure when neglected.
An good and effective program involves:
• scheduled monitoring and inspections
• bait and trap placement designed for food environments
• documented corrective actions
• facility-wide structural integrity checks
Pests bring pathogenic risk, physical contamination, and allergen issues. Keeping them out reinforces every other food safety control.
5. Allergen Control
Allergens continue to be one of the most common and expensive reasons products are pulled from the market, and the most recent recall reports from both the FDA and the EU show that this trend is not slowing down. These reports make one thing very clear, even well established operations can end up in recall situations when allergen management is anything less than precise. This is why allergen control programs must keep cross contact in check through smart separation and tightly managed handling processes.
Key components include:
• dedicated tools or color coding
• controlled changeovers
• validated cleaning methods
• accurate labels
• restricted ingredient flow
• allergen-specific training
Strong allergen controls reduce both regulatory risk and brand reputation risk.
6. Employee Training & Personal Hygiene Programs
Operators drive your day-to-day food safety performance. Even the strongest HACCP plan fails when staff do not fully understand their role in maintaining it.
Training programs should include:
• HACCP fundamentals
• CCP monitoring instructions
• allergen awareness
• sanitation duties
• illness reporting
• handwashing and hygiene expectations
Training must be ongoing, not a once-a-year checkbox. Consistent behavior equals consistent safety.
7. Equipment & Facility Maintenance
When equipment isn’t performing the way it should, hazards creep in fast. When it’s maintained properly, your controls stay stable, your process stays predictable, and your operators can trust the numbers they’re monitoring.
A solid maintenance program includes:
• preventive maintenance schedules that keep equipment running as intended
• controlled use of lubricants and chemicals to avoid contamination
• clear documentation of repairs and adjustments
• routine inspection of wear prone parts that can break, shed, or drift out of spec
• ensuring all surfaces and components remain cleanable and hygienically designed
Strong maintenance reduces the risk of foreign materials, temperature variation, and unexpected downtime. It also supports your HACCP system by making sure the equipment behind your critical controls is reliable every single day.
8. Traceability and Recall Readiness
Even with a strong HACCP system, incidents can still occur. Traceability gives you visibility across your entire product flow, and a well structured recall program ensures you can respond quickly, accurately, and with full confidence when something goes wrong. These two elements work together to protect both consumers and your brand.
Effective systems allow you to:
• trace ingredients one step back and one step forward
• identify affected lots within minutes
• remove product rapidly and accurately
• document actions for regulatory review
A recall program is something you never want to use, but if the day comes, it has to work smoothly. That’s why running a mock recall at least once a year matters so much. It gives your team a chance to practice the process, spot any gaps, and build confidence before it ever counts for real. A recall plan only truly works when everyone knows exactly what to do the moment it’s needed.
Why Prerequisite Programs Matter for HACCP?
Prerequisite programs give your HACCP system something solid to stand on. When sanitation, hygiene, allergen control, facility upkeep, and training are already working well, HACCP can focus on the truly critical hazards instead of getting pulled into everyday problems. Strong prerequisites make monitoring clearer, audits far less stressful, and daily operations more consistent across shifts. They create the stable conditions every HACCP plan needs. When these fundamentals are strong, the entire food safety system stays steady and dependable.
This leads us to HACCP Principles.
What Are the 7 HACCP Principles and How Do They Work in Real Production?
The seven HACCP principles form the structured, globally accepted method for controlling food safety risks across your entire process.
These principles guide how you identify hazards, select the steps that require strict control, set measurable limits, monitor performance, respond to deviations, verify effectiveness, and maintain the documentation that proves everything is working. While the principles themselves are universal, their implementation is entirely dependent on your product, your equipment, and your facility. That’s why understanding the context behind each principle is essential, especially when choosing CCPs or deciding what truly belongs in your HACCP plan.
The following breakdown uses real operational examples that reflect how manufacturers actually apply HACCP on the production floor, helping your team see not just what to do, but why it matters for day-to-day operations.

1. Conduct a Hazard Analysis
You review each process step and identify all potential hazards so you know which risks require control.
A HACCP plan begins by identifying every potential food safety hazard in your process and deciding which ones are significant enough to control. This step gives you a complete picture of the risks that exist in your production flow and guides all further decisions.
Hazards may include biological risks such as pathogenic bacteria in raw ingredients, physical risks such as foreign objects, chemical risks such as cleaning residues, and allergen risks created through cross contact. For each hazard you determine where it may appear, how severe the impact could be, and how likely it is to occur under real operating conditions.
A strong hazard analysis looks at:
• the source of each hazard and where it enters the process
• how likely the hazard is to occur and how serious the outcome would be
• which controls already exist and whether they are effective
• whether the hazard requires a CCP or can be managed through prerequisite programs
Common examples include poor hygiene, contaminated raw materials, inadequate cleaning practices, cross contact during changeovers, incorrect cooking or cooling steps, poor storage or ventilation, pest activity, and packaging materials that introduce foreign material risks.
2. Determine Critical Control Points (CCPs) in Food Safety
You identify the process steps that can fully prevent or reduce a serious hazard and keep the food safe.
Once you understand which hazards matter most, the next step is deciding exactly where in your process you can control them. A Critical Control Point is any step where you can remove a hazard or reduce it to a safe level. This is where HACCP becomes practical, because you stop treating every step the same and focus on the moments that truly protect the consumer.
A CCP is a step where:
• the hazard can be prevented, removed, or reduced to an acceptable level
• the control can be measured and verified
• the control is essential for safety, not just helpful
Your HACCP team reviews the hazard analysis and evaluates each processing step. This may involve scientific standards, regulatory requirements, your own process knowledge, or tools like decision trees. The aim is to identify the small number of steps where losing control would directly lead to unsafe food.
Typical examples include:
• validated cooking or pasteurization
• cooling within safe time and temperature limits
• hot or cold holding for ready to eat products
• metal detection where foreign material risk is real
• protecting product after the kill step
Not every control belongs here. HACCP works best when CCPs are limited to the steps that genuinely make the difference.
CCPs versus CPs
A CCP is essential for food safety and eliminates or reduces a hazard to a safe level.
A CP, or control point, is a general control step that supports good practice but is not the final safety barrier.
Example 1
Washing vegetables is a CP because it removes debris but does not eliminate all hazards. Cooking chicken to a validated internal temperature is a CCP because it destroys pathogens and nothing later in the process can fix a failure.
Example 2
A fresh salsa line identifies cold holding at the service bar as a CCP because temperature control directly affects pathogen growth. If it warms up, the product becomes unsafe. On the same line, rinsing produce before chopping is a CP. It is useful, but it is not the final safety step.
CCPs are few, focused, and critical. They are the moments where your food safety system truly prevents harm.
3. Set Critical Limits
You set the exact measurable values that keep each CCP safe and running within proven food safety boundaries.
Critical limits are the concrete numbers that tell you whether a CCP is truly under control. They define the specific minimums or maximums needed to keep a hazard at a safe level. These limits are never guesses. They come from science, regulation, or validated process data and create a clear line between safe and unsafe.
In practice, critical limits often relate to:
• time and temperature for cooking or cooling
• pH or water activity targets
• concentration levels for sanitizers
• holding temperatures for ready to eat food
A good critical limit has a few qualities. It is based on solid scientific evidence, it is measurable on the floor without confusion, and it applies the same way to every batch. Operators should know exactly what number they are aiming for and why that number matters.
When a critical limit is missed, control is lost and the product cannot move forward without evaluation. This is why limits must be tight and easy to verify. They protect you from guesswork and ensure your CCP does what it is meant to do.
Example
A refrigerated hummus line sets a critical limit of keeping product at or below 40°F because temperatures above that range allow bacteria to multiply quickly. If the line drifts warmer, the batch is held, the equipment is checked, and the team identifies what caused the rise. Staying within the limit is what keeps the product safe.
Critical limits are the backbone of CCP performance. They turn a theoretical control into something measurable, consistent, and defensible in any audit or investigation.
4. Establish Monitoring Procedures
You define how each CCP will be checked, how often, and by whom, so you always know if the process is under control.
Monitoring is how you confirm that a CCP is doing what it is supposed to do. It gives your team real time proof that the critical limit is being met and that the hazard is staying under control. Good monitoring is clear, consistent, and easy for operators to perform during normal production, not only when the plant is quiet.
Monitoring tells you:
• what needs to be checked at each CCP
• how the measurement will be taken
• how often the check must happen
• who is responsible for doing it
• how the results will be recorded and reviewed
Depending on the hazard and the step, monitoring might involve checking temperatures, measuring pH, using test strips for sanitizer concentration, watching a gauge or digital readout, or conducting a quick visual confirmation. Some processes use continuous monitoring such as automated sensors, while others rely on scheduled checks done by trained staff.
Clear responsibilities are essential. The person performing the monitoring must know exactly what the limit is, how to measure it correctly, and what to do if the result is out of range. All results need to be written down immediately. These records become part of your HACCP documentation and provide evidence that controls were maintained.
A good monitoring program also ensures equipment used for measurements is calibrated and reliable. If your thermometer is wrong, your CCP might look safe on paper while drifting out of control in reality.
Example
A chilled soup line assigns an operator to check product temperature every thirty minutes using a calibrated probe. The reading is written on the monitoring log, and the supervisor reviews the entries at the end of the shift. If the temperature rises above the critical limit, the operator follows the corrective action procedure and holds the batch.
Monitoring is how your CCPs prove they are working. Without it, even the best critical limits are just numbers on a page.
5. Identify Corrective Actions
You plan exactly what happens when a CCP goes out of control so unsafe food never continues in production.
Corrective actions are your “something went wrong, here is exactly what we do next” playbook. Even strong HACCP systems have moments where things slip. Temperatures rise, someone takes a reading too late, a tool is not calibrated, or a setting is accidentally changed during a busy shift. Corrective actions make sure these moments stay contained and never become a risk for consumers.
A solid corrective action plan explains:
• how to quickly bring the CCP back under control
• what happens to the product made during the deviation
• who is responsible for taking action
• how the event is recorded and reviewed
• how the root cause will be fixed to prevent repeat issues
Corrective actions are always written ahead of time for each CCP. When a deviation occurs, the team does not guess or negotiate the next step. They follow the plan, document what happened, and make sure no questionable product moves forward.
Corrective actions usually include:
• holding product until safety can be evaluated
• reworking or disposing of product that does not meet safety limits
• checking tools or equipment to confirm they are working properly
• retraining or clarifying procedures if the issue was human error
Everything must be documented. Auditors love this section because it shows how your operation behaves when things are not perfect. Good corrective action records prove control, transparency, and maturity in your food safety system.
Example
A cooled sauce line checks product temperature and finds it at 15°C instead of the required 4°C or below. The operator stops the batch immediately, verifies the reading with a calibrated thermometer, and alerts the supervisor. The product is placed on hold, evaluated, and discarded because it exceeded the safe temperature limit. The team then reviews what caused the warm-up and adjusts the procedure so it cannot happen again.
Corrective actions keep unexpected issues from turning into real hazards. They show that your team can follow the plan when things go right and respond effectively when they do not.
6. Implement Verification Procedures
You confirm that your HACCP system consistently works in real operations and continues to control hazards as your facility evolves.
Verification is how you keep your HACCP plan from becoming that polite document everyone nods at but nobody truly checks. It confirms that your controls are effective, your monitoring is accurate, and your people are applying the system the same way on every shift. Verification proves the plan performs on the production floor, not just during an audit walkthrough.
Verification has two major parts. First you validate the plan before it goes live to confirm the control measures truly work. After that you verify the system regularly to ensure it remains effective over time.
Strong verification programs include:
• validating that CCPs and preventive measures are scientifically sound
• calibrating thermometers, pH meters, scales, and any monitoring tool you rely on
• reviewing monitoring and corrective action records to confirm limits were met
• conducting internal audits to evaluate how well the plan is being followed
• performing product tests or environmental swabs when appropriate
• directly observing CCP monitoring and corrective actions during actual production
• reviewing supplier performance and certificates to verify upstream controls
• running mock recalls with your Supply Chain Department and suppliers and retailers to confirm traceability, communication, and decision making work when it counts
• confirming that deviations are investigated and improvements are applied
Verification is not a once-a-year chore. It must be scheduled, documented, and updated whenever your process, equipment, or ingredients change.
Reassessment is part of verification. At least once a year, or whenever you introduce new equipment, modify a recipe, adjust your layout, or experience an incident or complaint, the HACCP plan should be reviewed to ensure all hazards are still controlled effectively.
Example
A sauce facility validates its cooking step by proving the kettle consistently reaches the required temperature to reduce pathogens. During production, thermometers are calibrated weekly, supervisors observe CCP checks monthly, and monitoring logs are reviewed at the end of each shift. Twice a year the team performs a mock recall to verify that traceability data is accurate and complete. When a new heating system is installed, the cooking step is revalidated to confirm that the critical limit is still achieved.
Verification gives you proof that your food safety system works during real production, not only when someone is preparing the plant for an audit.
7. Maintain Documentation and Records
You document every part of your HACCP system so you can prove control, track issues, and respond quickly when needed.
Recordkeeping is the part of HACCP that quietly holds everything together. It captures the checks, decisions, corrections, and verifications that show your system is functioning in real operations, not just in theory. Without dependable records, you cannot defend your controls or trace an issue during a recall. With them, you have clear evidence of consistency and compliance.
Your documentation needs to cover the full system including:
• your HACCP plan and hazard analysis
• CCP monitoring results
• corrective action records
• verification and validation activities
• equipment calibration reports
• internal audits and inspections
• supplier and ingredient documentation
• training and competency records
• traceability and lot movement
• updates to procedures, ingredients, or equipment
Regulators expect accurate, complete, and easily retrievable records. Requirements like FSMA 204 and GFSI recognized schemes look closely at documentation because it reveals how reliably your operation manages food safety over time.
Strong documentation is not about paperwork. It is about visibility. When something goes off track, your records help you pinpoint what happened, contain the issue, and show you responded correctly. They also make audits smoother and allow supervisors to review trends instead of guessing.
Many teams now use digital food safety systems because paper, spreadsheets, and email attachments tend to disappear at the worst possible moment. This is where foodflou fits naturally into the workflow. The foodflou document control module keeps HACCP files, training logs, supplier records, and verification documents organized in one place. CAPA traceability is built in, so issues can be tracked, assigned, and resolved without chasing emails or losing evidence. You can even share actions or documentation requests directly with suppliers, which reduces risk and prevents gaps in communication.
To summarize, good recordkeeping turns your HACCP plan into a living, traceable system that stays reliable every day, not only during audits.
Practical Tips for Making HACCP Work on the Production Floor
HACCP succeeds when it is visible, simple, and baked into daily habits. Keep CCP instructions posted where the work actually happens so operators do not need to hunt for them. Make sure people understand why each control matters because numbers only stick when the purpose is clear. Use calibrated tools since guessing is not a control measure. Review the HACCP plan whenever equipment, ingredients, or processes change so the plan stays accurate. Keep prerequisite programs strong to avoid flooding your HACCP plan with problems that should be solved elsewhere. And choose CCPs wisely because HACCP works best when it focuses on the steps that truly protect consumers.
Why Use foodflou for Modern HACCP and Food Safety Management?
Most food safety teams are not held back by hazards, they are held back by disorganized documents, slow communication, and systems that feel like they were built for someone else. foodflou fixes that by giving you a modern platform that actually fits the way manufacturers work.
Everything lives in one place, from HACCP plans to CCP records, supplier files, training logs, verification activities, schedules, and CAPAs. You get full supplier visibility so you can see performance, missing documents, approvals, and risks clearly. NCRs and customer complaints can be logged, assigned, tracked, and closed with complete traceability. And every form in foodflou can be customized, so the system adapts to your process instead of forcing you into rigid templates.
It is also cost effective compared to traditional food safety software, which helps manufacturers protect margins while modernizing their food safety systems. Onboarding is fast and free, so your team can begin using the platform without long delays or heavy setup requirements.
You save time, gain visibility, and operate with far more confidence. If you want to see how this works in practice, schedule a demo and see foodflou in action. It takes minutes to get started and your team sees the impact immediately.
Final Thoughts on Building a Compliant HACCP Plan and Food Safety Program
A strong HACCP plan is more than a regulatory requirement. It is a practical way to keep operations steady, protect your brand, and ensure every batch meets the same high standard of safety. When your prerequisite programs are solid, your hazard analysis is honest, your CCPs are well chosen, and your monitoring and verification are consistent, HACCP becomes a powerful everyday tool rather than a stressful audit exercise.
With the right structure, the right culture, and the right digital support, your food safety program becomes smoother, clearer, and far more predictable. That is what modern manufacturing needs, and that is exactly what a well-built HACCP system delivers.
.png)
Frequently Asked Questions About HACCP
• What Makes a HACCP Plan Compliant?
A compliant HACCP plan is built on accurate hazard analysis, clearly defined CCPs, validated critical limits, reliable monitoring, documented corrective actions, ongoing verification, and complete records that prove control over time. It must also reflect how your process truly runs on the floor, stay updated as operations change, and align with regulatory and GFSI requirements. When all seven principles are applied correctly and supported by strong prerequisite programs, the plan meets compliance and performs consistently in audits.
• What is the first step in developing a HACCP plan?
You begin by assembling a multidisciplinary HACCP team that understands your products, processes, and operational risks.
•When should a HACCP plan be reviewed?
At least once a year, and any time you introduce new equipment, change ingredients, update processes, modify layouts, or experience food safety incidents.
• What is the difference between a CCP and a control point?
A CCP controls a hazard that must be eliminated or reduced to a safe level. A control point helps manage quality or general conditions but is not the final safety barrier.
• Why is HACCP important in food manufacturing?
It creates predictable control, reduces risk, prevents recalls, and is required for compliance with global standards like BRCGS, SQF, and FSSC 22000.
• Is a HACCP plan required for certification?
Yes. GFSI-recognized schemes and many regulatory agencies require a functioning HACCP plan and system.
• How often should monitoring equipment be calibrated?
Regularly, based on manufacturer recommendations and internal policy. In most facilities, this means weekly or monthly.
• How to use your HACCP plan in daily operations?
Use your HACCP plan as a daily guide, not an audit document. Keep CCP instructions visible at workstations, make monitoring simple and consistent, and record results as they happen. Use calibrated tools so readings are accurate. Follow corrective actions immediately when something goes off spec and make sure supervisors review logs each shift. Update the plan whenever processes or ingredients change. When everyone uses the HACCP plan this way, it stays reliable and keeps food safety steady across all operations.




.svg)

.svg)







.png)




.svg)



.png)



.svg)

